A global Pharma developed a mRNA-based vaccine for COVID-19. The storage temperature of the vaccine must be maintained at or below -20oC. The client needed a safe, multi-dose vial that eliminated breakage and maintained seal integrity throughout the cold chain. The client also needed a supplier that could scale-up manufacturing rapidly to meet the global vaccine demand.
SiO2 provided a 10mL vial for the Clients COVID-19 vaccine. The vial exceeds the Clients cold storage requirements and is currently supplying vials to two of the Clients CDMOs in the USA and Europe.
Through Operation Warp Speed, SiO2 increased vial manufacturing capacity over a 6-month period. SiO2 can supply 15-million vials/month. SiO2 qualified a second plastic resin for assured supply.
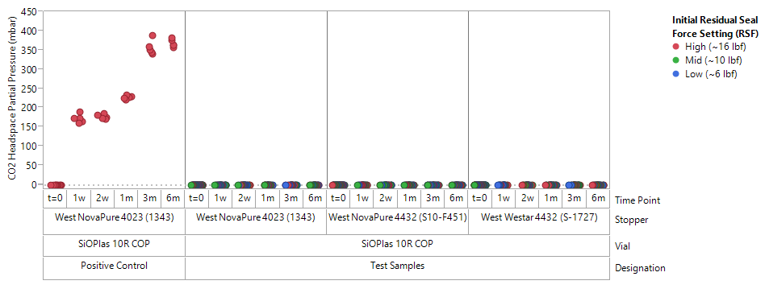
Seal Integrity Maintained at -800C for 6-months
Custom Designed 2.25mL staked needle syringe with a thicker barrel
No Vial Breakage down to -70°C Compared to Glass
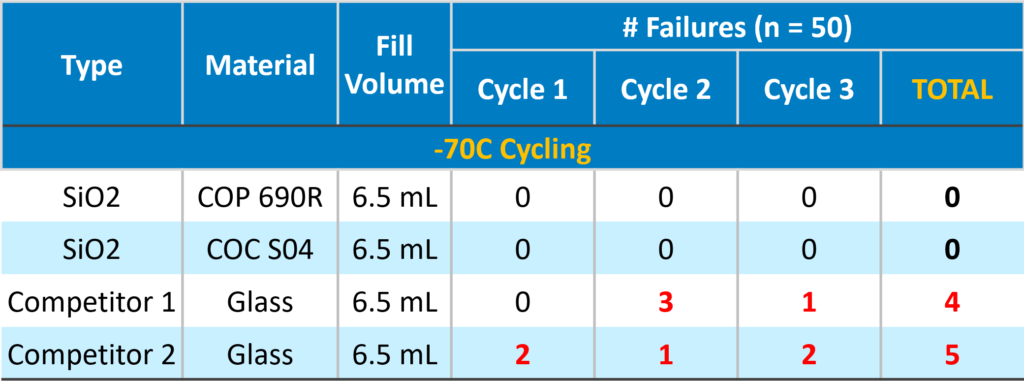
-70°C to RT (24 hr. soak at each temp, no ramp between)
We would love to discuss how SiO2 can be a solution for you. Contact us today.
© 2023 SiO2 Materials Science