A study was conducted by the Client that compared syringe performance and drug stability of the following syringe systems:
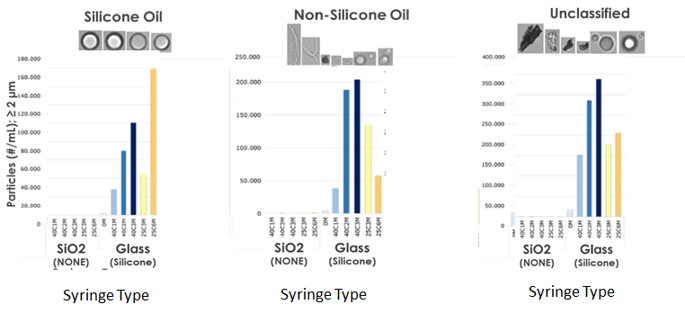
No subvisible particles larger than 2 microns found in biopharmaceutical formulations packaged in SiO2, lubricant free syringes (NONE) measured by micro flow imaging (MFI).
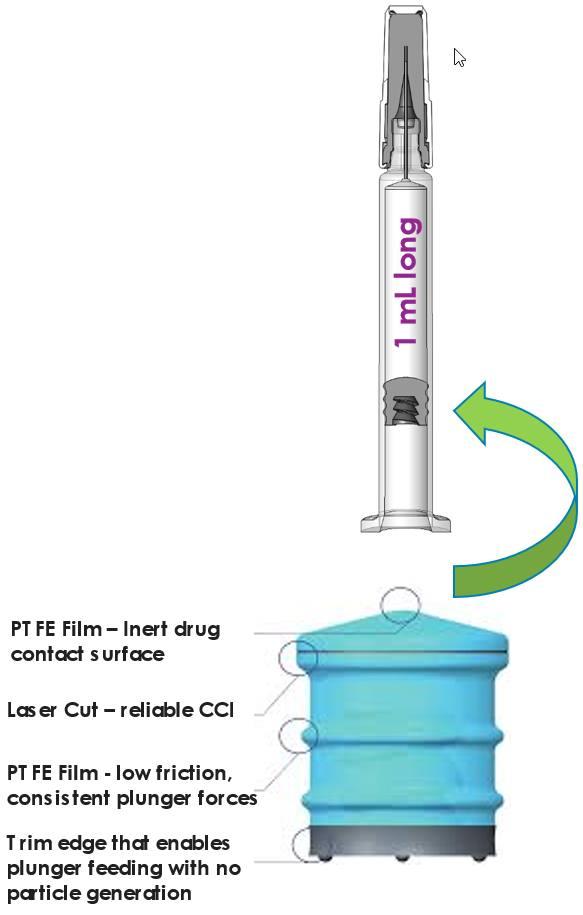
Self Lubricated Plunger
We would love to discuss how SiO2 can be a solution for you. Contact us today.
© 2023 SiO2 Materials Science